Phosphorus, sulfur and calcium: the key elements in calcium phosphates
An econometric analysis of price transfer from basic elements to finished goods
Published by Emanuele Altobel. .
Inorganic ChemicalsThe calcium phosphate industry represents a crucial sector for agriculture, chemistry and human health. These chemical compounds, containing calcium and phosphorus, are essential in numerous applications, including fertilizers, animal feeds and dietary supplements.
The European Union shows a substantial balance between imports and exports of phosphates (both calcium and dicalcium[1]). However, the EU is highly dependent on imports of the raw materials needed for their production. Among the main suppliers, Morocco plays a leading role, followed by the Gulf countries.
Calcium phosphate and dicalcium phosphate
This article explores the cost and price dynamics of calcium phosphate and dicalcium phosphate. The former is mainly used in the fertilizer industry, while the latter also finds application in the food and pharmaceutical sectors.
To understand the evolution of phosphate prices, it is essential to analyze the production process and the raw materials that constitute its key inputs. These raw materials can be traced to three key elements:
- Phosphorus: derived mainly from natural phosphate rocks.
- Calcium: naturally occurring in the form of limestones.
- Sulfur: while not present in phosphates, is essential for the production of sulfuric acid, which in turn is essential for synthesizing phosphoric acid.
The infographic below illustrates the chemical processes by which these three basic elements are transformed into calcium and dicalcium phosphates.
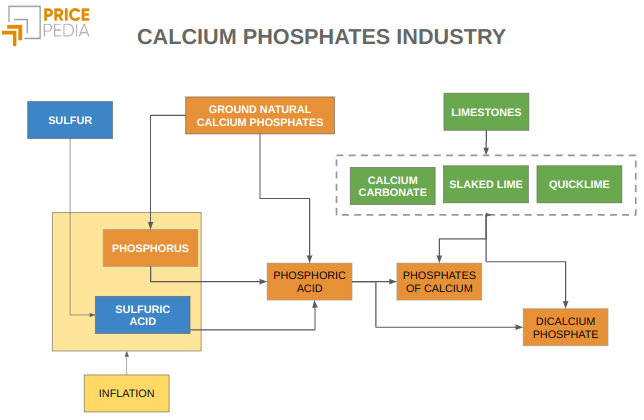
Calcium and dicalcium phosphates are produced by combining phosphorus (contained in phosphoric acid) with calcium (from calcium carbonate, quicklime or slaked lime).
Phosphoric acid, in turn, is produced by the reaction between sulfuric acid and phosphorus, contributed in the form of pure phosphorus or contained in phosphate rocks.
The central element in the phosphate production process is phosphoric acid, which is the most significant cost component. This is clearly illustrated in the graph below.
Price level of phosphoric acid and calcium phosphates.
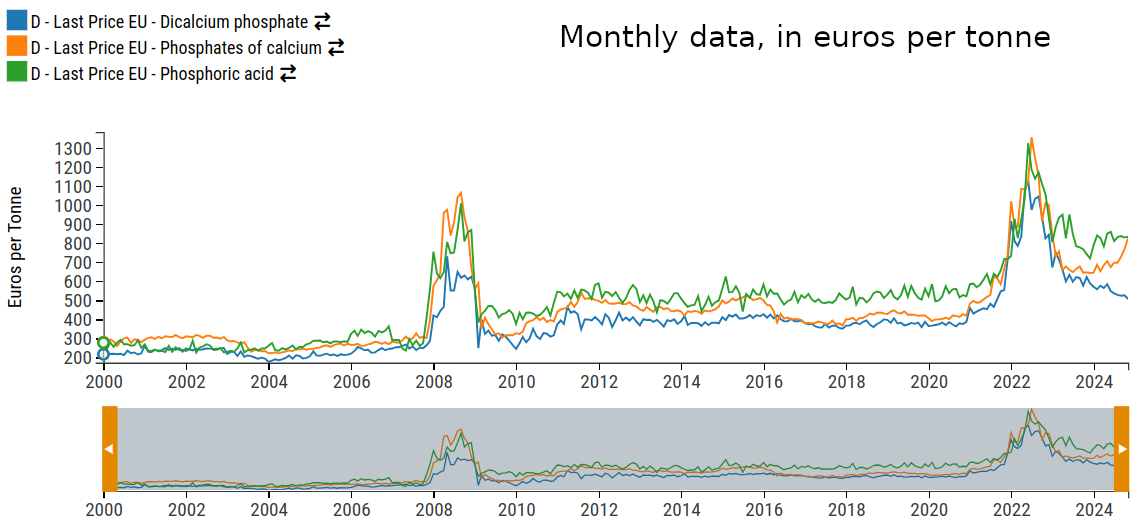
The graph shows that the input price tends to be higher than the output price. This phenomenon is explained by the fact that the amount of phosphoric acid required to produce one unit of phosphate is less than one unit. The difference is offset by the use of calcium carbonate, the price of which is significantly lower than that of phosphoric acid.
Do you want to stay up-to-date on commodity market trends?
Sign up for PricePedia newsletter: it's free!
An econometric estimation of price relationships along the supply chain
We estimated the price relationships linking various materials along the production chain, starting from basic elements and ending with finished products. The econometric models used are Engel and Granger type, with two separate equations: one for the long run and one for the short run.
The variables considered were transformed into logarithms, allowing the estimated coefficients to be interpreted in terms of elasticity. This approach allows immediate measures of the relative sensitivity of prices along the supply chain.
The following table shows the long-run elasticities along the production chain, calculated using the estimated coefficients of the long-run equation. All estimated coefficients are found to be highly significant. Moreover, tests performed on the estimates indicate statistically robust relationships, confirming that the reported elasticities reliably represent the true relationships between the market dynamics of different prices.
Long-term elasticity between prices along the calcium phosphate supply chain
| Phosphates of calcium | Fosfato dicalcico | |
| Calcium source (different depending on the product) | 0.25 | 0.20 |
| Source of phosphorus (phosphoric acid) | 1.04 | 0.78 |
| Phosphoric acid | ||
| Ground natural calcium phosphates | 0.19 | |
| Sulfur | 0.29 | |
| Sulfuric acid | 0.50 | |
| Phosphorus | ||
| Ground natural calcium phosphates | 0.40 | |
| Consumer price index | 1.14 | |
| Sulfuric acid | ||
| Sulfur | 0.48 | |
| Consumer price index | 1.35 | |
The data shown in the table highlight the predominant role of phosphoric acid, which affects between 80% and 100% of phosphate price changes. In comparison, the contribution of the calcium component is just over 20%.
Changes in the price of phosphoric acid, in turn, are determined about 50% by fluctuations in the price of sulfuric acid and the remainder by the price of phosphorus, which is present both in the form of pure element and phosphate rocks.
Finally, changes in the price of sulfuric acid are 50% influenced by fluctuations in the price of sulfur, with the remaining 50% attributable to general price inflation.
A summary of these numbers leads to an estimate that the respective price weights of calcium, phosphorus and sulfur in determining the price changes of calcium phosphates and dicalcium phosphates are 20%, 40% and 20%.
Conclusion
In this case study, we analyzed the production chain of calcium phosphates and dicalcium phosphates. After defining the chemical processes that link the different materials along the supply chain, we used these relationships to estimate the economic links between the prices of the various components.
The estimation results showed high robustness, allowing us to develop a reliable model. Such a model is able to accurately simulate how any shocks in commodity prices are transferred along the production chain, affecting the final prices of calcium phosphates and dicalcium phosphates.
[1] Dicalcium phosphate, also known as bicalcium phosphate or calcium hydrogen phosphate, is a chemical compound with the formula CaHPO₄. These three names are used in different contexts to refer to the same compound, which belongs to the calcium phosphate family